
Clinical Utility of Wood’s Lamp in Diagnosing Inflammatory Dermatoses, Cutaneous Infections, and Skin Malignancies
2025-05-28 15:20Introduction
The Wood’s lamp is a non-invasive, low-cost diagnostic device that emits ultraviolet A (UVA) light at approximately 365 nm, facilitating real-time evaluation of various dermatoses. First introduced by physicist Robert Wood in 1903, the tool remains relevant in dermatologic diagnostics despite a decline in its routine use. Historically recognized for its value in pigmentary and fungal conditions, recent literature underscores its broader role in inflammatory, infectious, and neoplastic skin disorders. This article systematically reviews current evidence on the Wood’s lamp’s clinical relevance in these domains.
Principles and Device Specifications
Classic Wood’s lamps utilize a mercury vapor bulb and a filter composed of barium silicate doped with nickel oxide, emitting UV light primarily within the 320–400 nm range, with an optimal output at 365nm. Modern handheld WLs may include magnifiers and predominantly emit UVA at peaks between 365 and 395nm. LED variants, while lacking magnification, are effective for fluorescence-based detection. Recent dermoscopy devices incorporate 365nm UV capabilities, allowing dermatologists to visualize diagnostic fluorescence patterns with greater precision.
Safety Considerations
Chronic UVA exposure from WLs may contribute to lenticular aging or cataractogenesis, though ophthalmologic consensus indicates minimal risk during routine use. Children, however, possess crystalline lenses with less UV filtration, rendering pediatric use more sensitive and necessitating eye protection.
Best Practices for Use Include:
Conducting examinations in a dark environment
Allowing mercury vapor lamps to warm up (~60 seconds) before diagnostic use (not required for LEDs)
Maintaining a working distance of 10–12 cm (or 30–40 cm for LEDs)
Avoiding pre-cleaning of suspected infected areas to preserve fluorophore concentration
Removing topical agents (e.g., sunscreen, makeup) before pigment disorder assessment
Recognizing that certain materials (e.g., ointments, lint, marker ink) may fluoresce and yield false positives
Diagnostic Role in Inflammatory and Autoimmune Dermatoses
Fluorescence occurs when UV photons excite skin-bound fluorophores, which then release energy in the visible light spectrum. Dermal fluorescence primarily stems from cross-linked collagen and enzymes like pepsin and collagenase, producing a blue-white luminescence. Because melanin absorbs UV radiation, areas of hyper- or hypopigmentation exhibit altered fluorescence intensity, enhancing contrast.
Wood's lamp also aids in detecting fluorescent byproducts of microbial metabolism (e.g., porphyrins) or exogenous substances. Table 1 outlines key clinical applications across dermatologic subtypes.
Table 1. Dermatologic Conditions Identifiable via Wood’s Lamp Examination
| Category | Condition | Fluorescence Characteristics |
|---|---|---|
| Inflammatory | Porokeratosis | White linear fluorescence at lesion edge |
| Morphea (plaque stage) | Darkened, well-defined areas | |
| Pigmentary | Progressive Macular Hypomelanosis | Red follicular fluorescence |
| Vitiligo | Bright blue-white fluorescence | |
| Melasma | Enhanced contrast (epidermal); none (dermal) | |
| Infectious | Tinea Versicolor | Yellow-green fluorescence |
| Erythrasma | Coral-red fluorescence | |
| Trichomycosis Axillaris | White or yellow luminescence | |
| Pseudomonas Infection | Bright green fluorescence | |
| Microsporum spp. | Green-blue fluorescence | |
| Trichophyton schoenleinii | Pale blue fluorescence | |
| Parasitic | Scabies | Bluish-white tunnels; greenish mite bodies |
| Neoplastic | Lentigo Maligna / Melanoma | Enhanced margin contrast under UV |
| BCC / SCC treated with ALA | Red porphyrin fluorescence | |
| Metabolic | Congenital Erythropoietic Porphyria | Pink fluorescence in body fluids and teeth |
| Porphyria Cutanea Tarda | Pink fluorescence in urine and stool | |
| Hepatoerythropoietic Porphyria | Similar to congenital erythropoietic form | |
| Erythropoietic Protoporphyria | Fluorescence predominantly in blood |
When examined under a Wood’s lamp, lesions of porokeratosis display a characteristic “diamond necklace” pattern — white fluorescent hyperkeratotic scales encircling a central blue-black core (Figure 1A and B). However, this fluorescence is transient and may not be consistently observed.
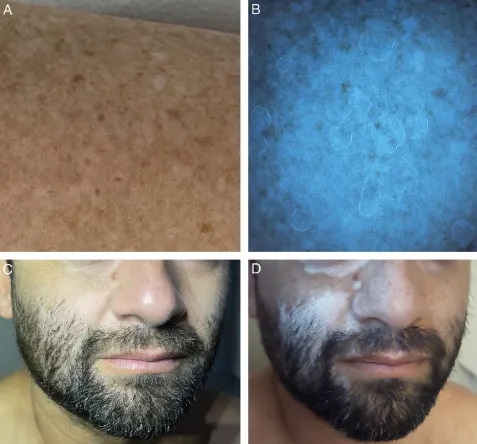
Figure 1. (A) Disseminated actinic porokeratosis. (B) Wood’s lamp examination showing the “diamond necklace” sign, with hyperkeratotic scales exhibiting white fluorescence. (C) Early-stage facial vitiligo. (D) Under Wood’s lamp illumination, the visibility of the depigmented area is markedly enhanced.
Porokeratosis
Wood’s lamp reveals a distinctive “necklace” fluorescence pattern: white margins with darkened central plaques. The fluorescent rim corresponds to the cornoid lamella—this presentation is diagnostically valuable, though transient.
Morphea and Hair Follicle Disorders
In follicular variants of porokeratosis, WL highlights follicular keratin plugs as punctate white signals. In early or subclinical morphea, WL may reveal dark, sharply demarcated patches not visible under ambient light, aiding early therapeutic intervention and longitudinal monitoring.
Applications in Pigmentation Disorders
WL excels in evaluating melanocyte-related anomalies. In vitiligo, the lack of melanin permits deeper tissue fluorescence, yielding a sharply defined blue-white glow. WL is also capable of detecting early or subclinical depigmented macules and quantifying treatment responses. Under UV365 dermoscopy, 40% of lesions reveal uniform fluorescence around hair follicles.
In tuberous sclerosis, small hypopigmented macules (“confetti lesions”) become more prominent under WL, often outperforming visual detection of the classic “ash-leaf” macules.
Melasma
WL assists in assessing melanin distribution depth:
Epidermal: Contrast is heightened under WL
Dermal: No contrast enhancement observed
Histopathological correlation remains variable; some reports affirm WL’s diagnostic value, while others dispute its ability to discriminate between dermal and epidermal melanin accurately.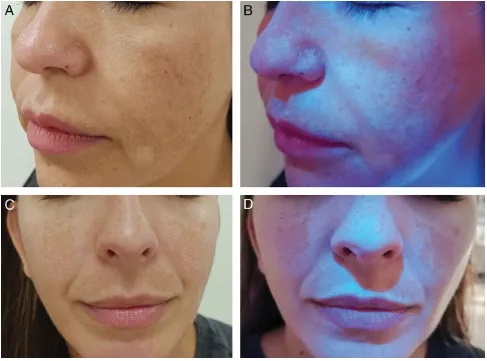
Progressive macular hypomelanosis is a pigmentary disorder caused by Cutibacterium acnes, a Gram-positive bacterium residing in hair follicles that produces coproporphyrin III. Under Wood’s lamp examination, the hypopigmented areas become more distinct, with red fluorescence observed within the follicles in the affected regions (Figure 3A and B). This diagnostic feature aids in distinguishing the condition from others such as tinea versicolor (yellow-green fluorescence), pityriasis alba (non-fluorescent due to irregular parakeratosis), post-inflammatory hypopigmentation, and idiopathic guttate hypomelanosis (blue-white fluorescence indicating dermal involvement).
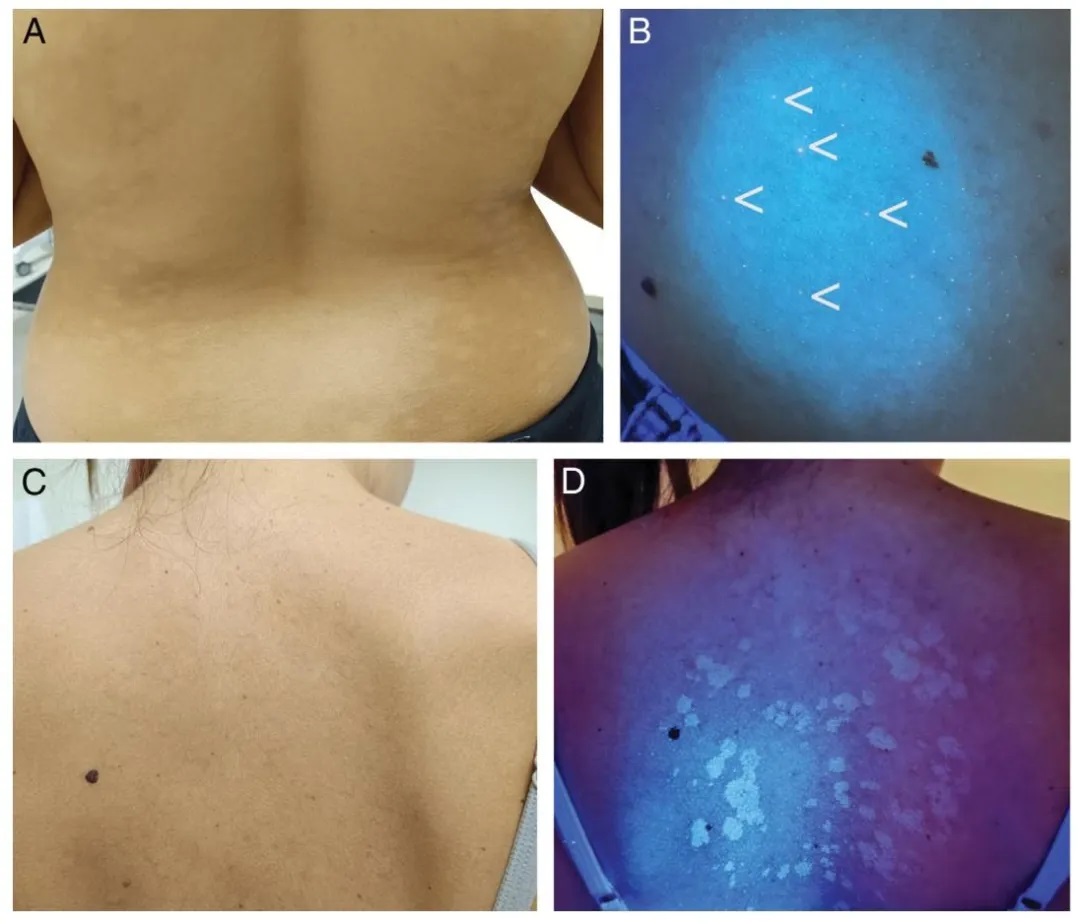
Figure 3.
(A) Progressive macular hypomelanosis.
(B) Under Wood’s lamp examination, red follicular fluorescence is observed in hypopigmented areas (more prominent to the naked eye than depicted in the image).
(C) Clinically subtle tinea versicolor.
(D) Yellow fluorescence under Wood’s lamp examination.
Progressive Macular Hypomelanosis (PMH)
Caused by Propionibacterium acnes, PMH presents with red follicular fluorescence due to coproporphyrin III accumulation. WL distinguishes PMH from tinea versicolor (yellow-green glow), pityriasis alba (no fluorescence), and idiopathic guttate hypomelanosis (bluish-white spots), improving diagnostic specificity.
Role in Infectious Dermatology
Erythrasma
A hallmark of Corynebacterium minutissimum, erythrasma shows a distinct coral-red fluorescence due to porphyrin production. This pattern helps differentiate it from non-fluorescent intertriginous dermatoses such as inverse psoriasis or candidiasis.
Dermatophyte Infections
WL helps identify specific fungal infections:
Tinea versicolor (Malassezia): Yellow-green fluorescence
Tinea capitis (Microsporum spp.): Blue-green hue
Favus (Trichophyton schoenleinii): Pale blue signal
Trichomycosis axillaris: White-yellow luminescence
Most Trichophyton species do not fluoresce
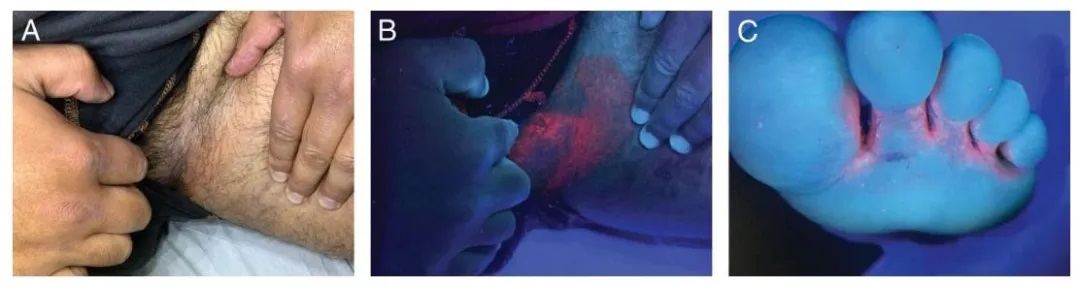
Figure 4.
(A) Erythrasma in the groin region.
(B) Coral-red fluorescence observed under Wood’s lamp examination.
(C) Erythrasma between the toes of the left foot, showing coral-red fluorescence under Wood’s lamp.
Pseudomonal Infections
Pseudomonas aeruginosa emits fluorescein, detectable as a vivid green glow under UV light. WL proves effective in identifying wound infections and green nail syndrome, allowing for prompt antimicrobial treatment.
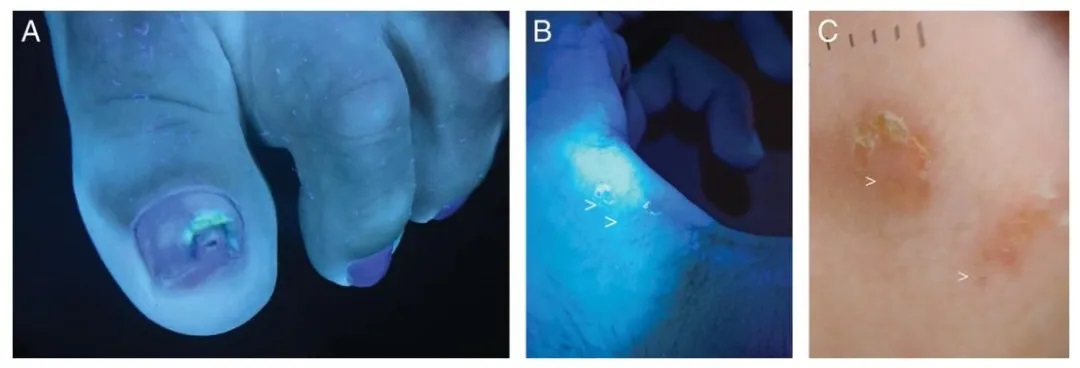
Figure 5.
(A) Green nail syndrome caused by Pseudomonas aeruginosa.
(B) Scabies burrow under Wood’s lamp (indicated by white arrow).
(C) Dermoscopic image of a scabies burrow (indicated by white arrow).
Use in Parasitic Dermatoses
Scabies
WL examination illuminates mite burrows as bluish-white linear tracks, with the mite body itself sometimes glowing white or green. UV365 dermoscopy further enhances mite visibility, aiding diagnosis in atypical cases.
Application in Cutaneous Oncology and Surgery
Lentigo Maligna
Delimiting LM margins is challenging due to subclinical extension. While current guidelines suggest 5–10 mm surgical margins, a large cohort study indicated that 15 mm may be required for a 97% complete excision rate.
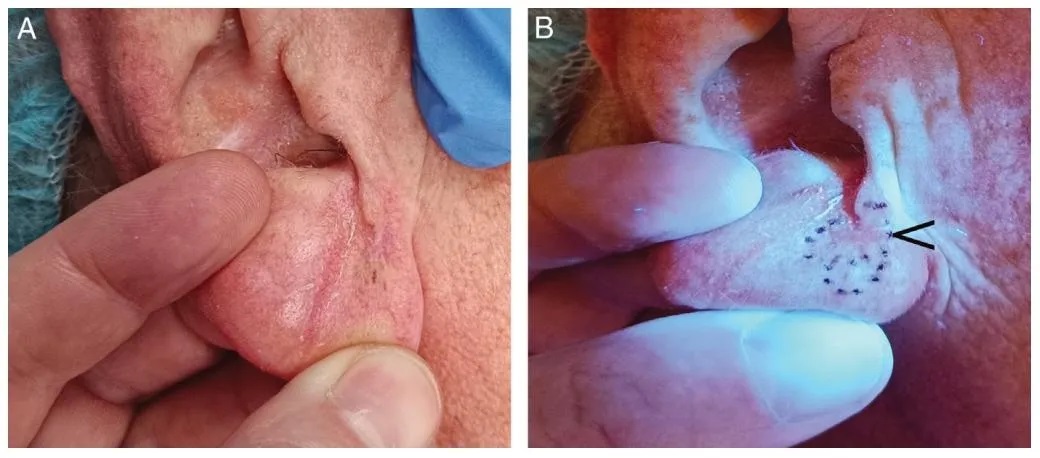
Figure 6.
(A) Malignant lentiginous nevus on the right earlobe, with poorly defined clinical margins.
(B) Enhanced margin delineation under Wood’s lamp, allowing for precise boundary identification during the first stage of Mohs micrographic surgery. The black arrow indicates the site of a previous biopsy, which is clearly visible under Wood’s lamp.
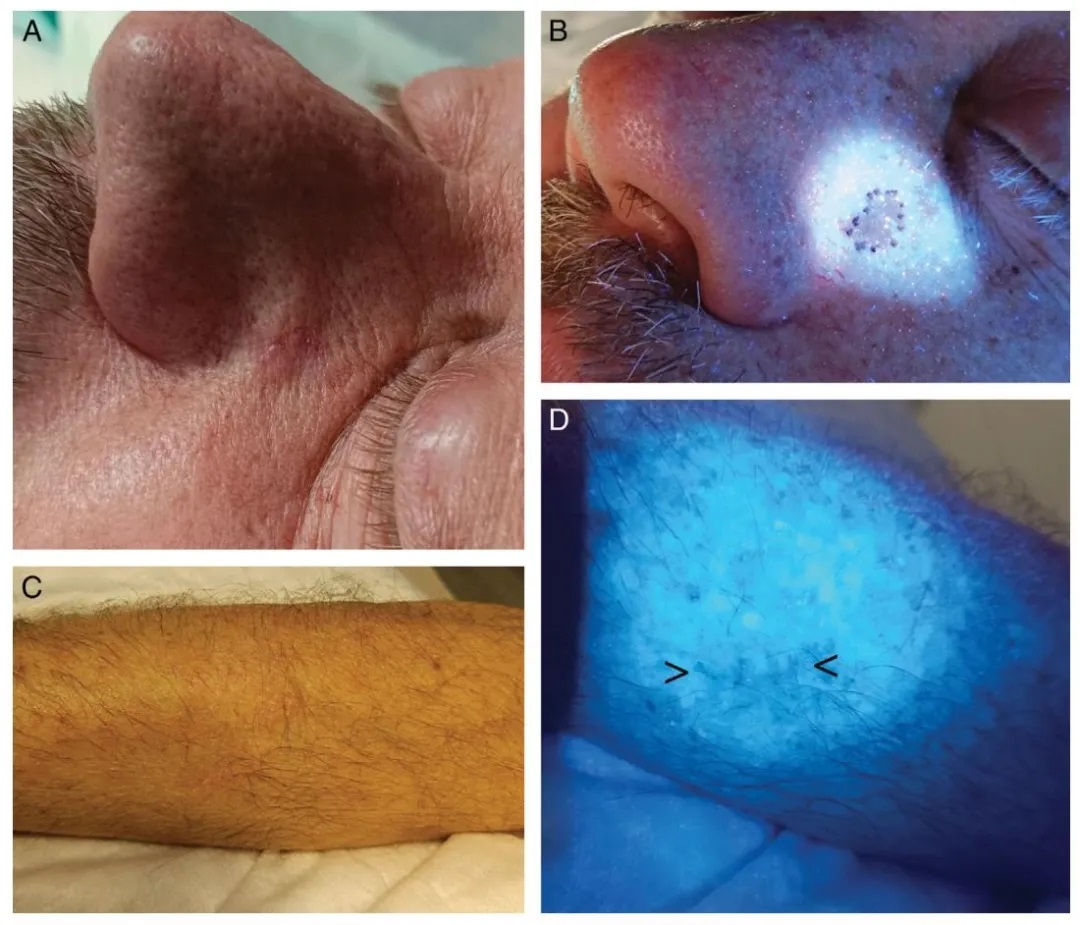
Figure 7.
(A) Basal cell carcinoma with indistinct margins on the left nasofacial fold.
(B) Preoperative margin demarcation using Wood’s lamp.
(C) Postoperative scar following excision of melanoma on the right forearm, with clinically unapparent extension prior to planned re-excision.
(D) Wood’s lamp easily highlights the scar (indicated by the black arrow).
Wood’s lamp accentuates melanin-rich tumor margins against surrounding fluorescent normal skin. Its preoperative use in Mohs surgery improves boundary assessment and may reduce recurrence. One prospective study demonstrated that WL-guided margin mapping aligned with final histological margins in 88% of cases when a 5 mm buffer was added beyond the clinically visible border.
