
Dermatology Journal Highlights|JEADV February 2025 Issue
2025-06-06 17:02Overview
This issue features the latest publications from the February 2025 edition of the Journal of the European Academy of Dermatology & Venereology (JEADV).
Literature Highlights | Table of Contents
Disease burden, management, and unmet therapeutic needs in bullous pemphigoid
DOI:10.1111/jdv.20313Cutaneous and systemic inflammation before and after whole-body topical treatment with 0.1% betamethasone or 0.1% tacrolimus in adult atopic dermatitis patients: a randomized controlled trial
DOI:10.1111/jdv.20258Glucocorticoid-induced myopathy in autoimmune bullous diseases: findings from an international study
DOI:10.1111/jdv.20149Topical crisaborole for dupilumab-associated facial dermatitis in atopic dermatitis patients: an open-label case series
DOI:10.1111/jdv.20281International consensus on methotrexate use in atopic dermatitis: an online Delphi study
DOI:10.1111/jdv.20271Precision diagnosis in pediatric dermatology: optimizing tinea capitis management through fungal PCR testing
DOI:10.1111/jdv.20147Buffalopox: an emerging human dermatologic disease
DOI:10.1111/jdv.19767Mohs micrographic surgery for invasive melanoma: a systematic review and meta-analysis
DOI:10.1111/jdv.20138“Barbie drugs”: social media marketing and awareness of melanotan
DOI:10.1111/jdv.20144Definition and clinical relevance of multidrug biologic resistance in psoriasis
DOI:10.1111/jdv.20133Cutaneous T-cell lymphoma and dupilumab: a retrospective matched cohort study on clinical features and treatment outcomes
DOI:10.1111/jdv.20141Oral monotherapy with tofacitinib, baricitinib, or upadacitinib for steroid-resistant vitiligo: a prospective case series
DOI:10.1111/jdv.20109Hepatitis B vaccine immunogenicity and treatment type in psoriasis patients: a retrospective cohort study
DOI:10.1111/jdv.20135Positive patch test results in biopsy-confirmed central centrifugal cicatricial alopecia patients
DOI:10.1111/jdv.20158Upadacitinib for treatment-resistant psoriatic arthritis
DOI:10.1111/jdv.20182Dermoscopic features of 35 pediatric pilomatricomas: a retrospective descriptive study
DOI:10.1111/jdv.20183Switching strategies between IL-23 and IL-17A inhibitors in psoriasis: inter-class switch superior to intra-class switch
DOI:10.1111/jdv.20192Intratumoral Daromun for nonmelanoma skin cancer: preliminary results of a Phase II non-randomized controlled trial
DOI:10.1111/jdv.20163Dermoscopy mimickers: facial mycosis fungoides versus pigmented actinic keratosis and pigmented lichen planus
DOI:10.1111/jdv.20185Disproportionality analysis of semaglutide- and tirzepatide-associated alopecia: a study based on the FDA Adverse Event Reporting System (2022–2023)
DOI:10.1111/jdv.20197Basophil and eosinophil recruitment in lesions and their association with autoimmune chronic spontaneous urticaria featuring basopenia
DOI:10.1111/jdv.20204Arthropathy-related adverse events of dupilumab: a systematic review
DOI:10.1111/jdv.20221Multicenter cohort study on the association between atopic dermatitis and cutaneous T-cell lymphoma
DOI:10.1111/jdv.20243Efficacy and tolerability of dupilumab in obese patients with moderate-to-severe atopic dermatitis
DOI:10.1111/jdv.20264Strategies to improve quality of life in cutaneous lymphoma patients
DOI:10.1111/jdv.20207
Featured Articles | Abstracts
1. Disease Burden, Management, and Unmet Needs in Bullous Pemphigoid [1]
Bullous pemphigoid (BP) is an autoimmune disease that primarily affects the elderly. It is characterized by tense blisters and intense pruritus, severely compromising quality of life. In recent years, the incidence of BP has been rising, particularly among individuals over 80 years old. It is often comorbid with neurological disorders, diabetes, and chronic kidney disease.
Current diagnosis relies on clinical manifestations and immunological testing (e.g., anti-BP180 antibodies). Treatment typically centers around corticosteroids; however, their side effects and risk of relapse limit efficacy. Special caution is needed in elderly patients due to polypharmacy and infection risks linked to immunosuppression. Biologics such as dupilumab have shown therapeutic promise in BP, but further research is required to confirm long-term safety and effectiveness.
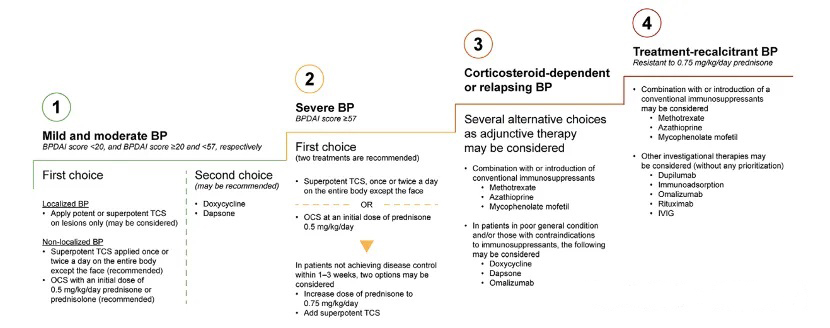
Figure 1. Stepwise treatment algorithm for BP
2. Cutaneous and Systemic Inflammatory Changes in Adults with Atopic Dermatitis Treated with 0.1% Betamethasone or 0.1% Tacrolimus: A Randomized Controlled Study [2]

Objective: To compare the effects of whole-body application of 0.1% betamethasone and 0.1% tacrolimus on skin barrier function and inflammatory biomarkers in adults with moderate-to-severe atopic dermatitis (AD).
Methods: A total of 36 AD patients were enrolled and received either 0.1% betamethasone ointment once daily or 0.1% tacrolimus ointment twice daily for two weeks, followed by four weeks of maintenance therapy. Disease severity was assessed using EASI scores. Natural moisturizing factor (NMF), cytokine levels in skin and blood, and phenotypic/activation status of peripheral T cells were evaluated.
Results & Conclusion: Both treatments significantly reduced AD severity and inflammatory biomarkers. Betamethasone was more effective in reducing both cutaneous and systemic inflammation, significantly lowering levels of IL-8, MMP-9, and other cytokines. Tacrolimus, on the other hand, showed superior improvement in skin hydration (as indicated by NMF levels).
3. Glucocorticoid-Induced Myopathy in Autoimmune Bullous Diseases: An International Study [3]

Objective: To investigate the risk of glucocorticoid-induced myopathy (GIM) in patients with autoimmune bullous diseases (AIBDs) undergoing glucocorticoid (GC) therapy, and to analyze incidence rates, associated risk factors, and the impact of cumulative dosage.
Methods: The study included 139 AIBDs patients treated between 2019 and 2023. GC toxicity was evaluated using the Glucocorticoid Toxicity Index (GTI). Muscle weakness was recorded along with severity grading. The effects of cumulative GC dose, average dose, treatment duration, and demographic factors on GIM were assessed.
Results & Conclusion: Muscle weakness of varying degrees occurred in 47.5% of patients. GIM was positively correlated with cumulative GC dose (Figure 2), while average dose and duration were not significantly associated. Age ≥50 years, male sex, and obesity (BMI >30 kg/m²) were significant risk factors. When cumulative GC dose was below 0.75 mg/kg/day, the incidence of weakness was low. The study recommends minimizing GC exposure and exploring exercise interventions and alternative therapies to mitigate GIM risk.
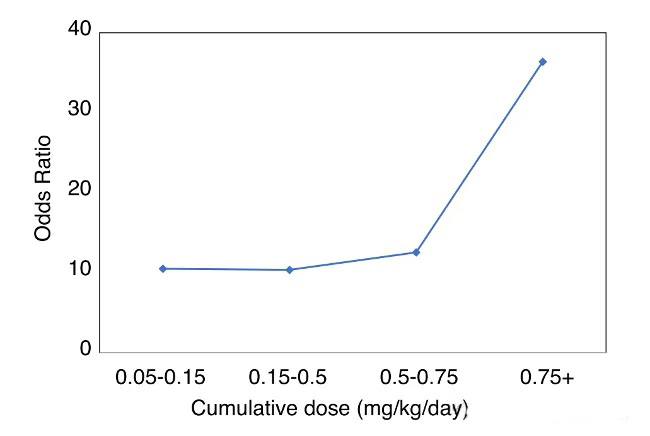
Figure 2. Odds ratio of myopathy development by cumulative GC dose
4. Topical Crisaborole for Dupilumab-Associated Facial Dermatitis in Atopic Dermatitis Patients: An Open-Label Case Series [4]

Objective: To evaluate the effectiveness of topical 2% crisaborole ointment for dupilumab-associated facial dermatitis (DFD) and explore its role in managing eczematous inflammation in the facial and cervical areas.
Methods: Eight AD patients who developed DFD during dupilumab therapy between January 2020 and December 2023 were enrolled. All received twice-daily application of 2% crisaborole for 4 weeks. Clinical symptoms, IGA score, EASI score, and BSA (body surface area) were recorded. Treatment-related adverse events were also assessed.
Results & Conclusion: All patients experienced significant improvement in facial and neck dermatitis symptoms after crisaborole treatment. IGA (p=0.008), EASI (p=0.012), and BSA (p=0.012) scores declined markedly, particularly with improvements in erythema, excoriation, and lichenification (Figure 3). Only one patient reported mild burning sensation; no serious adverse effects were observed. The average treatment duration was 13 weeks. Partial relapse was noted around 30 weeks post-discontinuation. The study suggests crisaborole is effective for alleviating DFD-related inflammation, potentially through inhibition of the Th1/Th17 pathway, although larger studies are needed to validate long-term efficacy and safety.
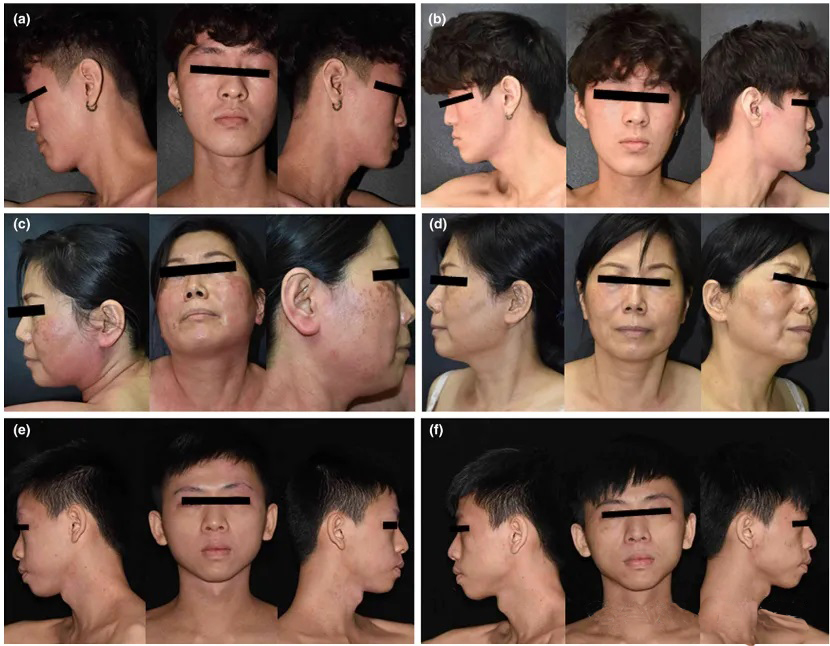
Figure 3. Lesion comparison before and after crisaborole treatment
